In the detection of breast cancer, MRI offers high sensitivity while
exposing the patient to no ionizing radiation. It is a painless exam, as
minimal or no compression is needed, yet it provides both morphological and
functional data.
MR is
commonly indicated to:
■ Identify clinically or mammographically
occult tumor
■ Stage and plan treatment
■ Detect recurrent tumor
■ Screen high-risk women
■ Evaluate the integrity of breast implants
It is also a valuable tool for guided breast biopsy.
1.5 and 3
TESLA MRI
Compared with 1.5T, 3T imaging has the inherent advantage of providing
a higher signal-to-noise ratio (SNR) and higher resolution or faster scans,
although there are trade-offs among these as well as certain limitations.
3T MR has a high specific absorption rate (four times that of 1.5T), decreased
B1 homogeneity, longer T1 relaxation time, and increased susceptibility
artifact. Despite these potential obstacles, a study from Kuhl et al1 showed that image quality was significantly higher at 3T than at 1.5T.
Clinical and technical considerations
The clinical role of contrast-enhanced MRI in the detection of breast
malignancy has been evaluated and established. Published research indicates breast MRI sensitivity of 77% and specificity of 77% at 1.5T.
Malignant tumors tend to have spiculated borders; they also tend to
show faster contrast uptake and washout than benign tissue. Thus, morphology
and enhancement kinetics are both important in determining
a lesion's status. High spatial resolution improves visualization of lesion
morphology, such as margins and internal architecture, whereas temporal
resolution of less than a minute is ideal for depicting enhancement kinetics.
Therefore, a complete breast MRI protocol must optimize and
balance both.
Imaging both breasts is essential for detecting occult cancer in the
contralateral breast, which occurs in approximately 3-5% of women with a
diagnosis of breast cancer. To avoid cardiac and respiratory motion artifacts through the
breasts, imagers should not use anterior-to-posterior phase encoding during the
axial or sagittal scan.
Most MRI breast imaging protocols for tumor evaluation include T2 as
well as T1 pre- and postcontrast sequences with the use
of fat-suppression techniques. T2 fat suppression may be useful for
differential diagnoses as cysts, effusion, and inflammatory processes all appear bright.
A T1-weighted sequence provides information on
morphologic information, such as fat and glandular structures. For
contrast-enhanced T1-weighted imaging, we use a 3D spoiled gradient echo sequence with a short repetition time, short
echo time, and shallow flip angle. The higher inherent SNR of 3D imaging allows thinner sections to be
acquired and increases overall spatial resolution in a given acquisition time
compared with 2D imaging. 3D imaging may also be more robust in dealing with B1 variations2 and
consequently allow for improved contrast-enhanced
breast images at 3T.
Fat suppression is important in detecting cancer as enhancing lesions
and fat both appear hyperintense
on postcontrast imaging.
A limitation of breast MRI is that dynamic contrast-enhanced (DCE)
breast MRI has high sensitivity but relatively low specificity.
Diffusion-weighted imaging (DWI) is based on the Brownian motion of water
molecules, which is restricted in tumors. In most benign lesions, there is
enough extracellular space and membrane permeability so that water molecules
can move freely between and around cells, which results in a high apparent diffusion coefficient (ADC) value. However, in most malignant
tumors, the cells are densely packed and water diffusion is restricted because
of reduced extracellular space and reduced membrane permeability. This results
in a low ADC value. If DWI is used in combination with other MR findings (margins and
kinetic analysis), the modality's sensitivity and specificity can be improved
to 92% and 86%, respectively.
Parallel imaging has improved the speed and quality of MRI over the
past decade. In conventional MR imaging, all
coils and amplifier channels collect the same image data. For parallel imaging
with a four-channel breast coil, two different receiver coil channels
simultaneously acquire different image data in the phase direction. Consequently, fewer k-space lines are acquired. Based on the sensitivity of
each coil, an algorithm combines wraparound images with conventional images.
Parallel imaging significantly reduces both the time of acquisition for a
postcontrast dynamic scan and distortion artifact in a
diffusion-weighted sequence.
Using breast coil, patient lying prone
■ Scout images
■ Axial T2 with fat suppression, both
breasts
■ Sagittal T1 3D, both breasts
■ Sagittal T2 with fat suppression, both breasts
■ Axial DWI using b =
1000 sec/mm2 to improve specificity
■ Precontrast axial 3D T1 with fat suppression, both breasts (mask images for subtraction)
■ Postcontrast axial dynamic multiphase 3D T1 sequence with fat-suppression (6 acquisitions)
■ Axial 3D T1
with fat suppression, both breasts (high-resolution scan)
■ Sagittal 3D T1 with fat suppression, both breasts (high-resolution scan)
Maximum intensity projection (MIP) provides an excellent overview of
image data and can steer the radiologist to suspicious lesions
in the breast.
Multiplanar reformation (MPR) helps pinpoint the location of enhancing
lesions in 3D. It allows the clinician to examine
lesions' internal structure and margins from a different perspective.
Creating signal-intensity time curves is the most commonly used
postprocessing tool. Placing an ROI on the most strongly and rapidly enhancing
pixels (typically five to 20
pixels) in a lesion creates a time-intensity curve. Kuhl et al
classified these curves according to their shapes as
type I, steady enhancement; type II, plateau of signal intensity; and type III,
washout of signal intensity. Of 101
malignant and 165 benign lesions described with
these signal intensity curves, they found that 83% of benign lesions were type I and 57% of malignant lesions were type III.
Patient preparation
When setting an appointment, except for urgent cases, premenopausal patients are informed that best results are obtained when the
examination is performed between days 7 and 14 after the first day of their menstrual
cycle to minimize hormonal influence on background enhancement. Before the
scan, we evaluate our patients for contraindications for MRI using
a standard questionnaire and safety screening procedure. Immediately prior to
the examination, patients put on a gown and pants to prevent possible metallic
artifacts from their clothing and to enable more comfortable positioning.
The technologist tells the patient of the approximate duration of the
scan and the importance of keeping still during image acquisition. The patient
is informed that she can communicate with the technologist via the intercom.
She is also provided ear plugs to protect against noise
created by the gradients.
Patient positioning
The patient is placed in a prone position on the breast coil with a
cushion placed under her head. The arms are positioned at
the sides of the body or above the head. One possible disadvantage is that the
axillary tail of each breast may not be fully covered by the breast coil.
Ensure that each breast hangs as deeply as possible within the coil opening
with the nipples centered and pointing straight down.
Contrast administration
Applications of either a single dose of 0.1 mmol/kg body weight or a double dose of 0.2 mmol/kg body weight have been described. To date, no strong data are
available to support an advantage for diagnostic
accuracy from double dosing. Therefore, because of concerns regarding
gadolinium deposition in tissue, which has been implicated in nephrogenic
systemic fibrosis, a single dose of 0.1
mmol/kg (0.2 cc/kg) is used in our hospital.
Prior to the examination, an intravenous angiocatheter, at
least 20 to 22
gauge, is inserted into the median antecubital vein, if possible. An injection
rate of 2 cc/sec of contrast followed by a 25 cc saline flush using a power injector
is preferred. Data acquisition starts right after the precontrast axial T1
fat-suppression sequence has finished with a 20-second delay.
Multiparametric
imaging of the breast at 1.5 T based on DWI, dynamic volumetric T1 Fat
Suppressed imaging, T2 volulmetric submillimeter FSE (GE Excite HDxT with 8
channel breast coil, OSIRIX 5.0.1 free edition, Mac OS 10.8.1). In this image:
sagittal reformat of 3D T2 FSE with 0.7mm slice thickness (3 minutes 20
seconds), DWI imaging (256x256, mm sl. thick.) and coloured ADC map for better
interpretation of the pathology and the different microstructure areas of the
pathology.
High
resolution and very thin slice (1.25 mm) fat suppressed DWI imaging for
consistent and reliable breast imaging for women with breast implants. The
combination of anatomical structures and wuantitative and functional data of
DWI offers great diagnostic fidelity even for the most difficult diagnostic
problems.
Fast
(2 minutes and 29 seconds for 256 slices), highly accelerated (R-factor = 4),
0.5 mm^3 pixel size, fast 3D gradient echo sequence with T1-weighting with
excellent fat suppression based on DIXON method offering fat and water imaging,
for consistent and reliable breast imaging for women with breast implants. High
anatomical detail offers diagnostic fidelity and low scan time offers clinical
integration of specialized volumetric imaging in everyday routine.
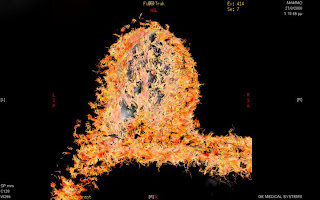
Reconstructed fiber tracking DTI offering a 3D view of the breast, the ducts and the relative position and infiltration of the pathology.
DTI was performed
with the following scan parameters: TR/TE = 10000ms/85ms, 2 NEX, 192 x 192
matrix, 28 x 28 cm FOV, 5mm slice thickness, and 3:00 min scan time. Diffusion
gradients were applied in 9 or 12 directions with b = 0 and 1000s/mm2.
Breast MRSI examination of a 42-year old patient. TR=1500 msec, TE=135
msec. The choline peak signal is visible. On the left picture the FWHM is more
than 14 Hz reducing dramatically the quality of the spectrum due to poor
shimming. On the right picture the effect of manual shimming is evident. Scan
time: 5 minutes. High SNR was measured in both cases exceeding 8 arbitrary
units.